[85] Table 2.8 (continued)
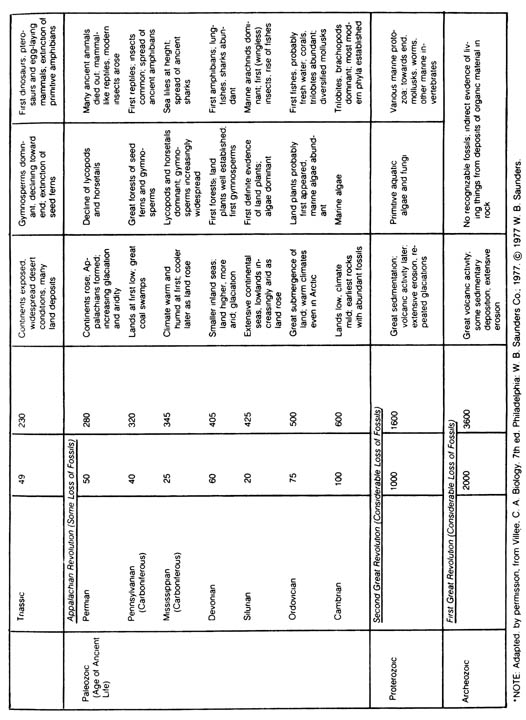
Table 2-8 Geographic time scale (400dpi, 850 kb)


2.2 Paleontological Evidence
Many evolutionists have claimed that the fossil record is conclusive evidence that evolution has occurred. However, if the paleontological evidence is closely scrutinized, the proof for evolution can be questioned.
2.2.1 Problems Encountered by Paleontologists
a) Incompleteness of the Fossil Record. As soon as an organism dies, its remains are subjected to three kinds of destructive forces: biological degradation, mechanical destruction, and chemical erosion.
Biological agents of degradation are ubiquitous, and there are hardly any natural habitats that are devoid of these agents. Scavengers devour all the edible parts of dead organisms. Smaller degradative agents, such as saprophytic bacteria and fungi, digest the remains. Even sturdy structures of dead organisms are subject to decay. For example, the structure of an oyster shell is made up largely of calcium carbonate. However, the structural matrix is held together by a network of organic degradable material. Therefore, as soon as the oyster dies, its shell is subject also to deterioration by biodegradation in combination with chemical erosion. This may account for the relative scarcity of empty shells in sea bottoms heavily populated by living shelled organisms other than the well preserved shells of the delicate protozoa, foraminiferans, and radiolarians. The burial of the organism immediately after death by sedimentation does not completely insulate the remains from biodegradation. Bacteria are found to be heavily concentrated in unconsolidated sediments in an aquatic environment.
Mechanical destruction may be an important abrasive factor if the early post-mortem history of an organism takes place in high-energy environments, i.e., areas where the action of wind, waves, and currents are [81] strongly felt. It was found in simulated experimental conditions that skeletons of bryozoans and calceraous algae were more sensitive to mechanical abrasion—such as tumbling with other pointed, unpolished pebbles—than were gastropods. Results such as this shed some light on the possible explanation of the relative fossil abundance of some organisms.
After the remains have been biodegraded and mechanically abraded, whatever is left, usually the skeleton, still has to withstand erosion and solution by chemical means. Chemical solution can take place at any time after the organism dies—even after the remains of the organism have become fossilized. The chances of a fossil being eroded by chemical solution depend on its chemical composition as well as that of its environment. Sometimes hollow cavities in rocks are all that remain after chemical erosion of some fossils, but these cavities are still recognizable and can be useful for paleontological classification.
Due to the operation of biological, mechanical, and chemical destructive forces, the parts of an organism that are most likely to be preserved are the hard, sturdy structures having high content of mineral instead of organic matter. Thus skeletons are preserved, whereas soft tissues are easily degraded or eroded. Therefore, the fossil record contains a biased selection of parts and types of organisms, according to their potential for preservation. Some knowledge derived from extinct mammals is based on fossils of teeth alone, due to their extraordinary durability. Because of the poor preservability of pigments, only very rarely do paleontologists have any fossil evidence that allows them to deduce the color of extinct organisms. The skeletons of various groups were also preserved to different degrees of completeness according to their chemical composition. For example, trilobite skeletons contain more pure calcium carbonate than those of crabs and are therefore more abundant.
An environment where burial by sedimentation or other physical forces is rapid is more likely to produce an abundance of fossils. However, areas being eroded by physical and chemical forces are less conducive to fossilization. Therefore, parts of the earth that are above sea level will be less apt to preserve fossils than areas below lea level because sea bottoms are constantly receiving sediments carried by rivers and streams. This may account for the overwhelming abundance of marine as compared with terrestrial fossils.
A biologically inactive environment is conducive to excellent fossil preservation. The spectacular preservations of vertebrates in tar pits and insects in amber represent the effect of completely biologically inert environments. Since these environments are not the normal habitats of the [82] preserved organisms, catastrophic events must have occurred during the process of fossilization.
Habitat influences the preservability of all organisms. Some organisms may be fossilized in an environment far away from their normal habitat by post-mortem transport meaning an organism dies one place and is carried to another. Pollens and spores of terrestrial plants are prone to be transported by wind. Remains of terrestrial plants or animals were carried by rivers into the ocean before they sank to the sea bottom and become fossilized. The effects of post-mortem transport on the distribution of fossils are hard to evaluate. However, paleontologists are extremely careful in trying to reconstruct the local environmental conditions according to the fossil record.
In summary, the fossil record is incomplete (1) and biased as to the parts and types of organisms preserved. Although large numbers of organisms lived during the earth's history, providing many fossils, the interpretation of the fossil record must be done with extreme care. Catastrophes may contribute to the fossil record although to an unknown degree. If catastrophes are shown eventually to be causing widespread fossilization, the principle of uniformitarianism (see I.2.1.1) has to be reevaluated. Paleontologists have to rely on large numbers of preserved samples to construct classification schemes. However, few fossils have been found of some organisms, and no conclusive information can be derived from scanty evidence.
b) The Somewhat Arbitrary Fossil Classification Scheme. As alluded to earlier (see I.1.2), the most objective basis for classifying the living world is the species concept proposed by Mayr as follows: "A species is an array of populations which are actually or potentially interbreeding and which are reproductively isolated from other such arrays under natural conditions." This concept cannot be applied to the classification of fossils because they simply cannot interbreed. Therefore, most paleontological classifications are based on observable characteristics of appearance, habitat, behavior, or geographic or stratigraphic occurrence. However, the relationship between different kinds of fossils cannot be established by morphological differences alone since phenotypic polymorphism (i.e., inherited variations in a population) is well known in the living world. Therefore, a presupposed phylogenetic relationship (the development or evolution of a kind or type of animal or plant) is the basic assumption of the modern paleontological classification scheme. To quote from a recent paleontology text:
If classification is to serve primarily for communication and identification, utility is the principal criterion for choosing one system over another. With the [83] rise of interest in evolution there has inevitably been a move toward using classification to express evolutionary relations also (2).Although the earliest taxonomists (e.g., Linnaeus and Buffon) recognized the possibility that limited changes may occur within a group of organisms after its creation, and there was speculation as to what taxa correspond to the "kinds" of creation recorded in Genesis 1 (3), modern taxonomists largely reject these interpretations. They adhere to an evolutionary presupposition and classify organisms according to their presumed phylogenetic relationships. The prominent taxonomist and paleontologist, George G. Simpson states the position of evolutionary taxonomist this way (4):
The principles of modern taxonomy are evolutionary and the approach to classification here taken is correspondly evolutionary or in a somewhat special sense phylogenetic. Many evolutionary processes can be observed in action, both in the field and laboratory, and so can extremely short segments of phylogeny. Those brief segments have great value for exemplification and for developing valid principles, but they have little practical application to classification beyond the lowest taxonomic levels, at best. Even the long series provided in many instances by paleontology are phylogenetic only by inference: the actual processes of reproduction and descent are not observed . . . . It is therefore time that evolutionary classification uses, for the most part, concepts and definitions for which the data are not directly observable. This is not a feature peculiar to taxonomy. It is shared in greater or lesser degree by most of the inductive sciences. They are not, on that account, less scientific, nor are their conclusions necessarily any less, or more, certain than if direct observation were possible. In an analogous way, although for quite different reasons, atomic physics deals with things that have never been directly observed, but no one would question the validity or utility of its interpretations in terms of particles and processes that are known only by inference.The claim made by Simpson that the inferential nature of taxonomic concepts is similar to that of atomic physics is not legitimate. While elementary particles are not directly observable, their existence and interactions can be empirically vindicated or falsified. For example, the law of parity, a theory dealing with weak interactions of atomic particles constructed by inference, was disproved by experimental findings (5). The existence of the J particle was discovered by empirical studies of interactions between light and lightlike particles (6). These empirical findings led to the establishment of new concepts in atomic physics, and the scientists involved were awarded the Nobel prize. However, although the hypothesis of the mechanisms of evolutionary change can he empirically vindicated or falsified (see I. 1.3) and the processes of microevolution [86] can be observed in the laboratory and nature, the inference that macroevolution "has occurred" in the past and that the phylogenetic tree can thus be derived can neither be empirically verified nor falsified. In a subsequent section (see I.3.3.2.a) it will also be seen that the mechanisms established empirically to account for microevolution are insufficient to account for macroevolution. Therefore, the extrapolation of the theory of microevolution, which Simpson indicated above as being documented empirically, into the still poorly explained areas of macroevolution is not well founded. The morphological data supporting the phylogenetic classification scheme at most can be taken as circumstantial evidence, and this evidence is not necessarily subject to an exclusively evolutionary interpretation.
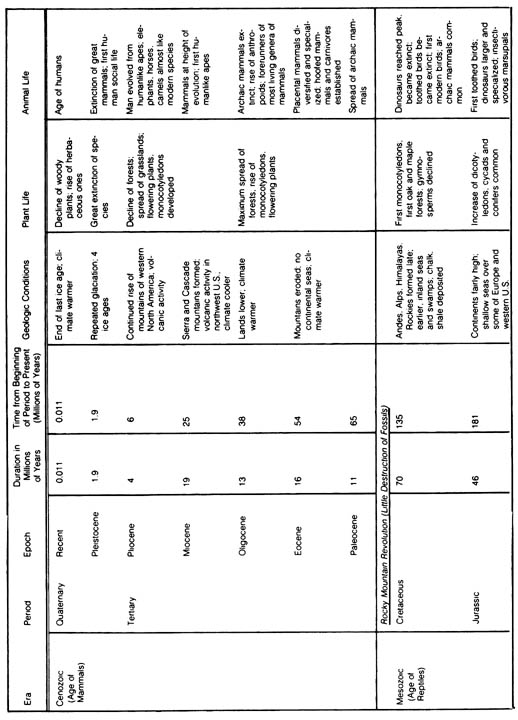
2.2.2 General Distribution of Fossils in the Geological Column. The most widely accepted scheme of distribution of biological taxa in the earth's history that has been constructed from the fossil record is shown in Table 2.8. It should be stressed that this table does not show the time of origin of different classes of organisms but instead only the time of dominance or first appearance in the fossil record. The origin of each major group of organisms can only be extrapolated from the fossil record.[84] Table 2.8. Geologic time scale.* 
[85] Table 2.8 (continued)
Table 2-8 Geographic time scale (400dpi, 850 kb)
Transitional forms possessing morphological characteristics of more than one major group are sought to bridge the gaps in the presumed phylogenetic tree. The scarcity or total absence, in many cases, of these transitional fossils has long been a mystery to paleontologists. However, there are cases of transitional fossils that may represent a gradual transformation between species, genera, subfamilies, and occasionally families. This evidence supports microevolution if one bears in mind that the classification of fossils is based essentially on morphological features defining the lowest taxa. However, in the higher categories where morphological features are clearly distinct there are systematic interruptions, as Simpson frankly admitted (7):
In spite of these examples (of transitional forms) it remains true, as every paleontologist knows, that most new species, genera, and families and that nearly all new categories above the level of families appear in the (fossil) record suddenly and are not led up to by known, gradual, completely continuous transitional sequences . . . Almost all paleontologists recognize that the discovery of a complete transition is in any case unlikely. Most of them find it logical, if not scientifically required, to assume that the sudden appearance of a new systematic group is not evidence for special creation or for saltation, but simply means that a full transitional sequence more or less like those that are known did occur and simply has not been found in this instance. Nevertheless, there are still a few paleontologists, and good ones, who are so impressed by [87] how much has been found that they conclude that most, at any rate, of what has not been found never existed, and there are scene neontologists, also some good ones, who accept this interpretation.Kerkut (8) has pointed out that missing licks exist between members of each of the following five major groups of organisms: (1) viruses, bacteria, and Protozoa; (2) Protozoa and Metazoa; (3)various invertebrate phyla; (4) invertebrate and vertebrate; (5) major groups of vertebrates. The transitional fossils that document evolutionary relationships within these groups are scarce or lacking.
The interpretation of the few transitional forms between major
groups are not without dispute. Fossil onychophorans, which resemble annelids
(segmented worms) except for their locomotive appendages, have been claimed
to be an annelid-arthropod interphylum intermediate (9)(see
Figure
2.7).
However,
while arthropods (e.g., trilobites) had reached a high development by lower
Cambrian time, onychophorans were just developing appendages for locomotion.
Moreover, extant onychophorans and arthropods show marked differences anatomically.
This leads to speculation of the polyphyletic (developed from more than
one ancestral type) origin of Arthropods
and the
Onychophora.
Alternatively,
the Onychophora
and
Arthropods
may have diverged from a remote
ancestral annelid stock (10).
[88] In another example, fossil monoplacophorans were believed to
be the transitional form between phylum Mollusca and phylum Annelida(11).
A recent monoplacophoran living fossil Neopilina has possible
internal segmentation similar to that of the annelid worms and arthropods.
However, it has the typical features of Mollusca that sharply discriminate
it from annelid worms and arthropods: restriction of an ectodermal cuticular
skeleton to the dorsal side, the muscular feet, and the pallial (mantle)
groove with gills (see Figure 2.8). Therefore, its transitional
status is inconclusive and more a matter of hypothesis and opinion.
Figure 2.8. Neopilina (Verma) sp., Rec., West Coast, Peru; ventral view with paired gills (X5.3). Reprinted, with permission, from Clarke, A. H., Jr.; Menzies, R. L. Science. 129 (April 17): 1026-1027; 1959. American Association for the Advancement of Science, Washington, D. C.
Figure 2.9. The oldest known Amphibian skeleton, Ichthyostega of late Devonian, about 3 feet long. Reprinted, with permission, from Romer, A. S. Vertebrate paleontology. 3rd ed. Chicago: The University of Chicago Press; 1966.
The fish-amphibian transition Ichthyostega (12)
has many features shared by the advanced bony fish crossopterygian and
the primitive amphibian labyrinthodont (see Figure 2.9). However, three
aberrant features of Ichthyostega
have puzzled evolutionists. (1)
The intertemporal bone in the skull is absent in Ichthyostega. This
is presumably primitive in the amphibian, being retained by later labyrinthodonts
but absent in more advanced amphibians. (2) The cheek region, which is
still rather [89] flexibly articulated with the skull roof in some Carboniferous
forms, is already firmly consolidated in Ichthyostega. The movability
of this basal articulation is considered a primitive amphibian feature
and is not present in more advanced forms. (3) Ichthyostega, crossopterygians,
labyrinthodonts, and all of the modern higher vertebrate classes have the
arch
vertebra
or its modified form, while many of the early Palaeozoic amphibians and
all the modern amphibians have the husk vertebra. (The arch type
of vertebra is characterized by two sets of ossified arch structures formed
in the central region of the vertebra-the anterior ones termed the
intercentra
[hypocentrum]
and the posterior, the pleurocentra
[Figure 2.l0c, d]. The husk
vertebra [Figure 2.33], on the other hand, is characterized by a single
structure often spool shaped and pierced lengthwise by a hole for the notochord
[Figure 2.10, a, b].) The noted absence of transitional forms linking the
fin of the crossopterygian and the [90] foot of Ichthyostega
is
also perplexing in the attempt to determine the relationship between the
two groups. It is thus difficult to see the links between crossopterygian,
Ichthyostega,
and
modern amphibians. Therefore, the conclusions drawn from this fossil are
very tentative.
The interpretation of another fish-amphibian transitional fossil Elpistostege is uncertain also. The only available evidence, a skull-roof fossil from the early upper Devonian period, shows no trace of the transverse break behind the parietal bones that in crossopterygians is associated with the bipartite braincase. However, there is an absence of any postcranial skeleton that may bear fins or legs (13).Figure 2.10. The vertebrae of Paleozoic lepospondyls, (a, b); and the vertebrae of labyrinthodonts, (c, d). c, articulation for capitulum of rib; ic, intercentrum; n or na, neural arch; p, pleurocentrum; t, attachment for tuberculum of rib. Reprinted, with permission, from Romer, A. S. Vertebrate paleontology. 3rd ed. Chicago: The University of Chicago Press; 1966.
The amphibian-reptile intermediates Seymouria and Diadectes
exemplify
the lack of definitive skeletal distinctions among the fossil amphibians
and reptiles (14) (Figure 2.11). Modern reptiles can
be distinguished from living amphibians by their bony structure, e.g.,
reptiles have only one condyle in the skull, modern amphibians, two; reptiles,
typically have five toes in the manus, whereas modern amphibians have four
or fewer; the sacrum in reptiles includes at least two vertebrae, modern
amphibians only one. However, the fossil evidence of amphibians and reptiles
is not clear-cut, for primitive Paleozoic reptiles share so many of the
skeletal characteristics of the earliest amphibians that it is almost impossible
to tell where the boundary lines are between the two classes.
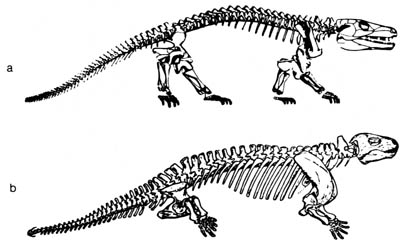
Figure 2.11. The seymouriamorph. (a) Seymouria, an early Permian seymouriamorph; (b) Diadectes, a highly specialized seymouriamorph of the early Permian. Maximum length about 10 feet. Reprinted, with permission, from Romer, A. S. Vertebrate paleontology. 3rd ed. Chicago: The University of Chicago Press; 1966.
The major definite character of reptiles is the amniote egg that
they lay [91] on land. This type of egg, similar to a bird's egg, contains
a large supply of nourishing yolk. The reptilian amniotic egg containing
an amniotic cavity filled with fluid provides an aqueous environment equivalent
to the aquatic environment of free-living larva (tadpoles) of amphibians.
Thus, the amniotic egg helps explain the absence of the tadpole stage that
is present in amphibians. However, it has not been possible to determine
whether or not fossil reptiles were amniotic. It is also of interest to
note that both Seymouria and Diadectes appeared in the early
Permian era (approximately 280 million years ago); however, the oldest
known reptile, Hylonomus was found in early Pennsylvania rock (approximately
320 million years ago). It appears that the transitional forms may have
arrived 40 million years too late to beget the first known reptile!
Archaeopteryx has been cited frequently as the transitional type between reptiles and birds (14). It has a birdlike skull and wings with feathers (Figure 2.12). The reptilelike features are represented by clawlike appendages, the possession of teeth, and the long vertebral column that extends into the tail. The flying power of this organism was presumably slight, for the wingspread is much less than that of the poor fliers among modern birds.
Since most skeletal features of birds can be matched by some archosaurian reptiles, feathers have been considered the only distinctive feature of birds. Feathers are essential to birds for insulation against loss of body heat, and this contrasts greatly with reptiles since they are cold-blooded. Therefore, Archaeopteryx was definitely a bird. However, the presumed small sternum, the primitive reptilian structure of wing bones, and especially the long tail set Archaeopteryx apart from most modern birds, requiring a separate subclass to represent it. However, some of the reptilian structures of this fossil are shared by some modern-day birds. For example, the juvenile stages of Opisthocomus hoatzin of South America (13) and Touraco cory thaix of Africa (15) possess claws, and both are fliers.
Diarthrognathus (Figure 2.13), a late Triassic fossil,
has been claimed to be a transitional form between reptiles and mammals.
This form possesses a quadrate-articular joint that is a feature characteristic
of tritylodonts, the last survivors of the most advanced group of the mammallike
reptiles Therapsida. Interestingly, a minor part of the same joint
is a squamosal-dentary contact, a more mammalianlike feature. However,
Diarthrognathus
lacks
the dental specialization of the tritylodonts; thus, its transitional status
can be questioned.
Figure 2.12. Fossil (below) of the primitive bird Archaeopteryx, and a restoration (above) or model depicting the appearance of this primitive animal. Courtesy of The American Museum of Natural History, New York.
The skeletal remains of many "transitional" forms, such as Diarthrognathus,
are
fragmentary. Furthermore, the demarcation between the reptilian [92] and
mammalian structures is becoming blurred as knowledge about each group
increases. The diagnostic characteristics of the class Mammalia
essentially
reside in the soft anatomy and physiology that cannot be determined from
skeletal remains. Therefore, the classification of mammalian fossil according
to skeletal features is tentative. In addition, the almost simultaneous
appearance of Diarthrognathus (late Triassic era) and the first
known mammal fossil (Triassic-Jurassic boundary) leaves little time for
the evolution of mammals from this presumed transitional form.
In summary, the transitional forms cited above are subject to various interpretations. It seems premature to base phylogenetic trees of organisms on these forms for which we have only fragmentary morphological evidence. More specifically, L. de Nouy, an evolutionist, commenting on the status of Archaeopteryx, has made a very succinct statement concerning the establishment of "true links" between major groups. While recognizing the morphological similarities of Archaeopteryx to reptiles and birds, he nevertheless concluded:
By link, we mean a necessary stage of transition between classes such as Reptiles and Birds, or between smaller groups. An animal displaying characters belonging to two different groups cannot be treated as a true link as long as the intermediary stages have not been found, and as long as the mechanisms of transition remain unknown (16).
Figure 2.13. Skull of the advanced therapsid Diarthrognathus; original about 1.5 inches long. Abbreviations for this skull: a, angular; ar, articular; bo, basioccipital; d, dentary; e, epipterygoid; eo, exoccipital; f, frontal; j, fugal; m, maxilla; p, parietal; per, periotic; pt; pterygoid; q, quadrate; sq; squamosal. Reprinted, with permission, from Romer, A. S. Vertebrate paleontology. 3rd ed. Chicago: The University of Chicago Press; 1966.
[94] It is sufficient to conclude that even if the "missing links"
can eventually be found (a proposition on which no one can have assurance),
the paleontological data can be used only as circumstantial evidence that
has to be examined by inference instead of by experimental observations.
References 2.2
1. Raup, D. M.; Stanley, S. M. Principles of paleontology. San Francisco: Freeman; 1971 (chapter 1).