


2.5 Evidence of Comparative Structure and Function
There are many similarities in comparative structure and function among
all living organisms. These similarities have been used by evolutionists
to support their thesis. This section reviews comparative structure and
function evidence by dividing it as follows: comparative cellular structure
and function, comparative gross anatomy, and comparative embryological
development. [129]
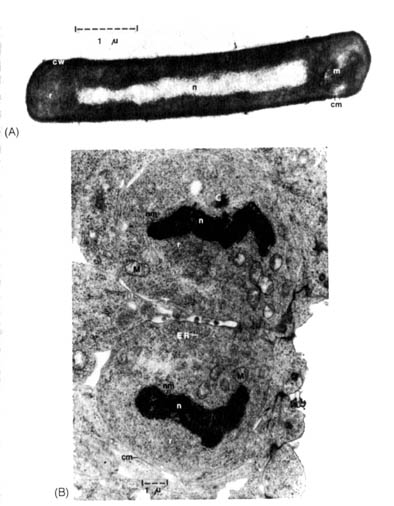
Figure 2.30. Electron micrographs of thin sections of
procaryotic and eucaryotic cells. (A) A bacterium, Bacillus subtilis,
that
has a typical procaryotic cell structure. It is surrounded by a relatively
dense cell wall (cw), enclosing the cell membrane (cm). Within the
cell, the nucleus (n) is distinguishable from the cytoplasm, densely
filled with 70S ribosomes (r). A membranous structure, the mesosome
(m),
is also found at one pole of the cell. (B) Two dividing nerve cells
from a three-day-old-chick embryo have a relatively undifferentiated eucaryotic
cell structure. These nerve cells lack cell walls outside their cell membranes
(cm).
Recognizable
structures in these cells that are not found in procaryotic cells are:
mitochondria
(M), endoplasmic reticulum
(ER), centriole
(C)
(part of the mitotic apparatus), 80S ribosomes (r), and the nucleus
(n)
surrounded
by a nuclear membrane (nm). These cells are in the terminal stage
of mitosis that will be followed by the complete separation of the two
cells and the reformation of the nucleolus in the nucleus. (Electron micrograph
(B)
is courtesy of Dr. John Sechrist, Department of Biology, Wheaton College,
Wheaton, IL.)
[130] 2.5.1 Comparative Cellular Structure and Function. All
living organisms are composed of small compartmentalized units called cells.
Cells are units of life because they can perform essential functions that
are indispensable for the survival of the whole organism. Cellular respiration
provides the energy necessary for the activities of the organism. Cellular
division and differentiation bring about the growth and development of
the organism. Many cells can exist individually as free-living unicellular
entities, such as bacteria or protozoa. Others constitute the building
blocks of multicellular organisms. For example, in an adult human being,
there are approximately one hundred trillion cells differentiated to form
different parts of the body.
With the advent of electron microscopy, it was apparent that cells could
be divided into two basic types according to the structure of the nucleus,
namely, eucaryotes and procaryotes (Figure 2.30). Eucaryotes
include mosses, liverworts, ferns, higher flowering plants, and all animals
that are characterized by multicellular tissues and systems involving extensive
differentiation of cells. Lower forms such as algae, protozoa, and fungi
(many are microscopic) are also eucaryotic. Algae, protozoa, and fungi
may be unicellular or multicellular. Multicellular forms may show little
or no differentiation of cells and tissues. Procaryotes, on the other hand,
consist of two main groups, the ubiquitous unicellular bacteria and blue
green algae (Monera). Table 2.14 compares the basic differences between
eucaryotic and procaryotic cells, and Figure 2.30 gives microscopic documentation
of the differences.
Table 2.14. Comparison of eucaryotic and procaryotic cells.
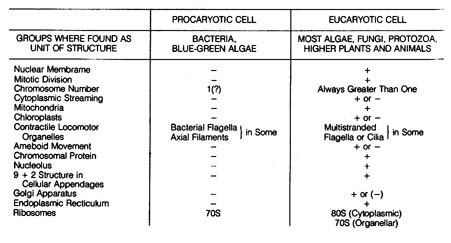
Table 2.14 400dpi 172k
[131] Although cellular functions are basically the same in the two
cell types (see. I.2.6), they are mediated in different ways.
For example, cellular respiration in the procaryote occurs in the plasma
membrane (cell membrane) while the corresponding reaction site in a eucaryote
is in the inner membranes of the cytoplasmic organelle, the mitochondrion.
The plasma membrane in some procaryotes is also the site of photosynthesis,
whereas the process in eucaryotes occurs in the chloroplast, a cytoplasmic
organelle. The similarities of the mitochondrial and chloroplast organelles
in the eucaryotic cell to an entire procaryotic cell are striking. They
are membrane-bound structures that contain DNA, RNA, and the same kind
of ribosomes. This has led many biologists to speculate on the evolutionary
origin of the two organelles. Figure 2.31 summarizes some of the current
thinking. The prevailing hypothesis is that certain bacteria came to be
permanently associated (in symbiosis) with precursor procaryotic amoeboid
cells, thus establishing the first true eucaryote.
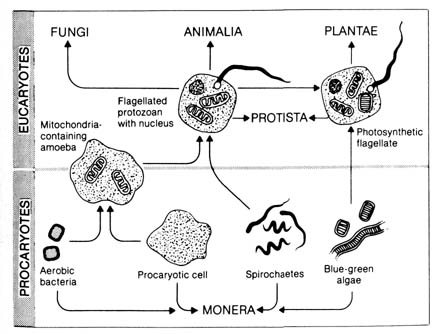
Figure 2.31. The evolution of eucaryotic cell types through
symbiosis. The membrane-bounded organelles that characterize eucaryotic
cells may have originated in symbiotic relationships among procaryotic
cells. Reproduced, with permission, from Stephens, G. C. Biology.
New York: John Wiley & Sons; 1974. © 1974 by John Wiley &
Sons.
[132] The evolutionary interpretation of the relationship between procaryotes
and eucaryotes is, to say the least, strained. There is no direct evidence
to link the two types of cells. It is also legitimate to suggest that the
two types have separate and independent origins. Therefore, evidence for
evolution based on cellular structure and function is purely circumstantial.
2.5.2 Comparative Gross Anatomy. The anatomical features of many
vertebrates follow more or less similar patterns. These patterns are used
as evidence for a common ancestor. Some of the frequently cited patterns
are the development of homologous structures, the development of
vertebra, the evolution of the heart, and the existence of vestigial
organs.
a) Homologous Structures. Homologous structures are defined as
those that can be traced to the same embryonic origin and are similar in
basic structure and development. They may not be used for the same function,
but their similarities are said to indicate a common ancestry. Figure 2.32
shows the bones of the forelimbs of the frog, lizard, bird, human, cat,
whale, and bat. The homologous nature of the structures seem to be evident
because of the similar arrangement of the bones in each member of the group.
Structural variations represent adaptations of each member to a particular
mode of life. Each structural variation is thought by evolutionists to
be built from a common ancestral form by the process of natural selection.
Evolutionists have based their conclusions about the common genetic
origin of the above organisms on the basis of anatomical similarities.
However, after examining this evidence, we cannot eliminate the possibility
that similar structures were created by God independently from a master
design with variations suitable for each group of organisms, according
to its mode of life.
b) Development of Vertebra. The vertebra of crossopterygian bony
fish and some of the earliest amphibians, the so-called arch vertebrae
(see
I.2.5.2.b),
consisted of a large anterior, medium wedge-shaped element that was incomplete
dorsally, termed intercentrum (hypocentrum), and two smaller, intersegmental,
posteriodorsal elements called pleurocentra
(Figure 2.10). This
configuration is also termed rachitomous.
The various types of vertebrae found in the different groups of vertebrates
has been compared and schemes constructed to show possible evolutionary
relationships. Figure 2.33 shows one possible evolutionary sequence of
the vertebra from crossopterygians and amphibians to modern amniotes. The
main line of evolution seems to involve the progressive reduction of the
hypocentrum accompanied by the progressive enlargement of the pleurocentrum
until finally the pleurocentrum takes over completely in the higher forms.
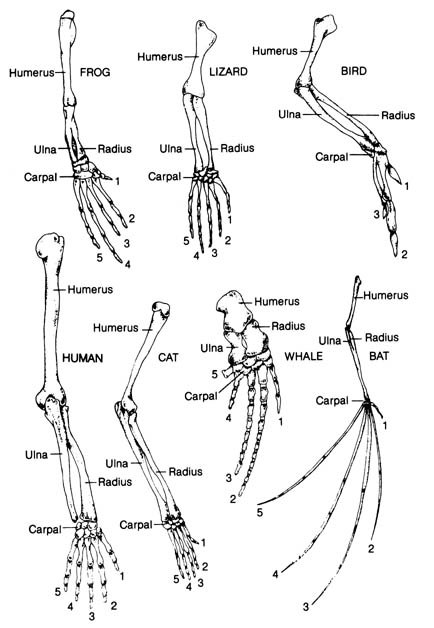
Figure 2.32. The bones of the forelimbs of a frog, lizard,
bird, human, cat, whale, and bat, showing the arrangement of the homologous
bones in these superficially different structures. Adapted, with permission,
from Villee, C. A. Biology. 7th ed. Philadelphia: W. B. Saunders
Co.; 1977. © 1977 by the W. B. Saunders Co.
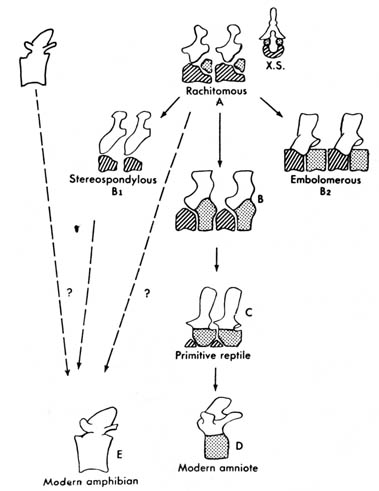
Figure 2.33. Comparison of vertebrae of primitive tetrapods
and modern amniotes. The rachitomous type (shown also in cross section,
X.S.) occurred in crossopterygians and in the earliest amphibians. B
is from a labyrinthodont in the reptile line. B1 and B2 are
from other labyrinthodonts. Whether the modern amphibian centrum represents
a hypocentrum (diagonal lines) or a pleurocentrum (stippled) is not certain.
The unmarked part of the vertebra is the neural arch. Adapted, with permission,
from Kent, G. C. 4th ed. Comparative anatomy of the vertebrates. St. Louis:
C. V. Mosby Co.; 1978. [134]
Vertebral changes for Stereospondyli and Embolomeri,
two extinct suborders of primitive amphibians, follow a different pathway.
In Stereospondyli the pleurocentrum is lost, and the hypocentrum
takes over completely. In Embolomeri both the hypocentrum and pleurocentrum
are retained, each expanding to equal size (Figure 2.33).
[135] The vertebral structure of modern amphibians, however, poses an
interesting question to the evolutionists. It is the so-called husk-type
vertebra and consists of a single structure somewhat spooled in shape and
often pierced lengthwise in the upper part for the passage of the notochord.
This type of vertebra was also shared by the extinct primitive amphibian
subclass Lepospondyli, which existed during the Paleozoic time (Figure
2.10a, b). Since the commonly accepted sequence of vertebrate evolution
is crossopterygian to amphibian and labyrinthodonts to modern tetrapods,
it is difficult to see how the modern amphibian could have evolved in light
of the similarities of its vertebra with an extinct subclass of primitive
amphibian that did not enter the main stream of tetrapod evolution (1).
(See discussion on fish-amphibian transition in I.2.2.2.) Therefore,
the evolutionary interpretation of the vertebra is somewhat unsatisfactory.
c) Evolution of the Heart. The circulatory systems of all vertebrates
are very similar. The principal differences reside in the heart. Figure
2.34 shows the heart structure of the fish, frog, reptile, bird, and mammal.
The fish heart is composed of four chambers aligned in series (linear
configuration): sinus venosus, atrium, ventricle, and conus (Figure 2.34a).
Veins collecting blood from the body drain into the sinus venosus. The
contraction of the atrium and ventricle forces the blood into the ventral
aorta that leads into the gills. Gas exchange occurs in the gills, and
the now-oxygenated blood is distributed to the body via the dorsal aorta.
Blood is circulated through the heart once during a gas- exchange cycle.
The fish's blood circulation is presumably less efficient than that of
land animals because blood passes only once through the heart for each
complete cycle through the body. However, since the rate of exchange of
dissolved oxygen in the water with the carbon dioxide in the blood is slow
due to the meager solubility of oxygen in water, the linear configuration
of the heart functions well for the fish.
The amphibian heart has a left and a right atrium separated by a partition
(Figure 2.34b). Venous blood enters the ventricle via the right atrium,
whereas blood oxygenated in the lungs enters the ventricle via the left
atrium. There is a tendency for the oxygenated and less oxygenated venous
blood to mix in the ventricle. However, venous blood from the right atrium
tends to enter the ventricle first and is followed by the oxygenated blood
from the left atrium. A spiral valve in the corms helps to guide the less
oxygenated blood into the pulmonary arteries upon initial ventricular contraction.
Continued ventricular contraction forces the more oxygenated blood into
the aorta. The aorta then carries the oxygenated blood into the different
parts of the body while the pulmonary [136] arteries deliver the blood
to the lungs for gas exchange. Blood is returned to the left atrium via
the pulmonary vein.
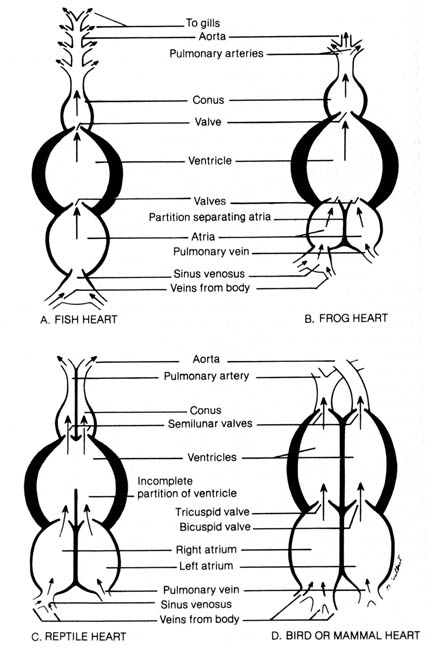
Figure 2.34. Diagram comparing the structures of vertebrate
hearts. Adapted, with permission, from Villee, C. A. Biology. 7th
ed. Philadelphia: W. B. Saunders Co.; 1977. © 1977 by the W. B. Saunders
Co. [137]
In the amphibian the circulatory system is more advanced than in the fish
because contractions of the heart can deliver the less oxygenated blood
to the lungs while pumping the more oxygenated blood through the aorta
to the body tissues. Due to the mixing effect in the ventricle, a red blood
cell could pass through the heart several times before completing a gas
exchange cycle in the lungs. However, the respiratory capacity of the thin,
moist amphibian skin helps to compensate for failure of all the deoxygenated
blood to be shunted to the lungs after the first pass through the heart.
Thus, the lungs and thin skin enable the amphibian to exist on land and
in the water.
In reptiles a more efficient circulatory-gas exchange system is needed
because of the thick cornified skin that prevents any significant skin
respiration. Most reptiles have a partial partition in the ventricle so
that the mixing effect of oxygenated and deoxygenated blood can be minimized
(Figure 2.34c). Alligators and crocodiles have the thickest and most cornified
skin and possess a complete ventricular partition. In all reptiles the
sinus venosus is less prominent than in the amphibians.
Birds and mammals have the most specialized hearts with two atria and
two ventricles; each chamber is completely separated from the others by
thick muscular walls (Figure 2.34d). In contrast with the reptiles, the
cornus and sinus venosus are absent. Oxygenated and deoxygenated blood
never mix. Deoxygenated blood enters the right atrium and passes to the
right ventricle. It then goes to the lungs, is oxygenated, and returns
to the left atrium. The blood then passes to the left ventricle and enters
the systemic circulation via the aorta. The circulatory pattern is an efficient
high-pressure system. This may account for the high metabolic rate in both
birds and mammals and the maintenance of a regulated body temperature.
Evolutionists have attributed the development of the structural differences
in vertebrate hearts to the creative role of natural selection, a process
that selects genes that can produce the structures best adapted to the
mode of living of each group of organisms. Alternatively, these structural
patterns, reflecting a common design with variation, can equally be attributed
to a divine Creator.
d) Vestigial or Rudimentary Organs. Many apparently functionless
structures in advanced animals and plants are thought to be the remnants
of once useful organs that have fallen into disuse because of natural selection.
These structures are assumed to be in the process of being eliminated.
At one point, there were thought to be up to 180 vestigial organs in [138]
humans alone; however, the number has been dwindling as the functions of
these organs are slowly being discovered.
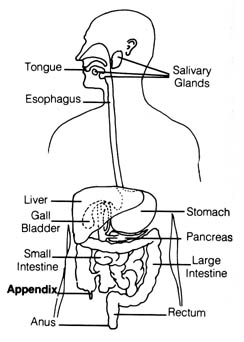
Figure 2.35. The appendix in the human digestive system.
The most frequently cited vestigial organ in humans is the vermiform
appendix (Figure 2.35). It can be surgically removed without any apparent
ill effect to the body. However, there are good indications from the studies
of the appendix in rabbits that this organ functions as part of the immune
system. In one experiment using the rabbit, total body irradiation with
shielding of the appendix resulted in restoration of normal antibody synthesis
after a short initial lag of three to four days, whereas the irradiation
on the appendix alone did not affect the antibody synthesizing capacity
of the rabbits (2) (Figure 2.36). Appendectomy alone
induced a slight suppression in antibody synthesis thought to be insignificant.
Another set of experiments suggested that the appendix in rabbits may be
responsible for the recovery of their capacity to manufacture blood-bound
lymphocytes after neonatal thymectomy (3).
The structure of the appendix in rabbits is quite different from that
in humans. However, it is reasonable to assume that the vermiform appendix
in humans probably also has the function of a lymphoid tissue that is responsible
for the replenishment of a damaged immune system. After all, most concepts
in immunology are derived first from animal studies [139] before they are
found to be applicable to humans. Therefore, the appendix in humans is
probably an important secondary source of the immune response, and it cannot
be treated as a useless vestigial organ without considering this possibility.
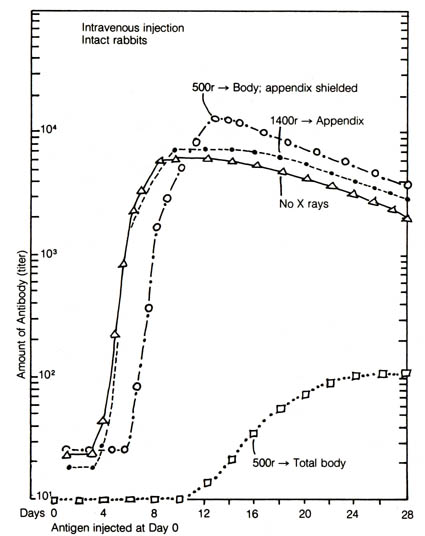
Figure 2.36. Mean antibody curves, showing the effect
of shielding of irradiation of the appendix as compared to totally irradiated
and nonirradiated rabbits. Note the complete protection to peak titer by
appendix-shielding as compared to the nonirradiated rabbits. Adapted, with
permission, from Sussdorf, D. M.; Draper, L. R. Journal of Infectious
Diseases. 99:135; 1956. The University of Chicago Press.
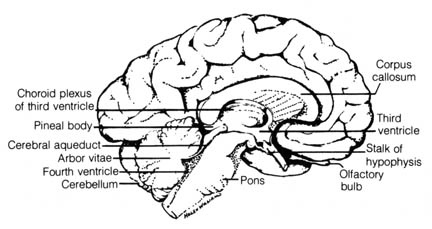
Figure 2.37. Human brain, left half, sagittal section.
Reprinted, with permission, from Francis, C. C.; Martin, A. H. Introduction
to human anatomy. 7th ed. St. Louis: C. V. Mosby Co.; 1975. [140]
The pineal body in humans (Figure 2.37) was once thought of as a
vestige of the mid-dorsal third eye found in the skull of extinct primitive
chordates. It is now known that the pineal body is responsible in living
primitive vertebrates for the synthesis of the hormone melatonin (4)
that regulates the distribution of skin pigments. In mammals, however,
it functions in the regulation of sex-hormone secretion. Studies of the
time-measuring system of the sparrow indicate that the pineal body seems
to affect the parts of the brain that may be involved in some aspect of
the biological rhythm phenomenon (5). Thus, the pineal
gland has been shown to be far from functionless in the body of vertebrates.
The plica semilunaris in humans (Figure 2.38) and other mammals, regarded
as
a vestigial structure of the nictitating membrane or third eyelid in birds,
was found to be structurally and functionally different from the nictitating
membrane (6). This fold in the human eye may serve as
an added protective barrier to bar foreign substances from entering the
eye. The sticky mass formed in the corner of the eye may be formed by the
fatty substance secreted by the plica semilunaris to trap dirt, preventing
it from damaging the eye.
The vestigial coccygeal vertebrae (Figure 2.39) of the coccyx in humans
seems to serve the function of helping to support the abdominal viscera
in light of its being bent forward toward the abdomen. It is also possible
that the coccyx provides attachment for a muscle that controls the process
of elimination of feces.
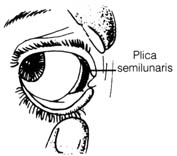
Figure 2.38. Plica semilunaris in humans. Reprinted, with
permission, from Villee, C. A. Biology. 7th ed. Philadelphia: W.
B. Saunders Co.; 1977. © 1977 W. B. Saunders Co.
The status of other structures formerly thought to be vestigial is now
[141] being challenged, including human male nipples, tonsils, and certain
portions of the whale skeleton. The interpretation that the nipples in
the human male are inherited from an ancestor in which they were functional
is being questioned (6). The tonsils were once considered
useless but are now known as part of the lymphoid mass that traps infectious
agents (7, 8). Another doubted claim
deals with the existence in whales of transitory teeth and small bones
embedded in the flesh, the bones supposedly corresponding to the pelvis,
femur, and tibia. However, the conclusion cannot be made from this evidence
that the whale descended from a tetrapod ancestor with functional teeth
and a normal skeletal structure (6). Some of these structures
in the whale are believed to be important in the animal's developmental
process.
In summary, although there are still body structures in higher animals
and plants that appear to serve little if any function, further research
is likely to show the importance of many of these structures. In order
to demonstrate the vestigial nature of many structures with unknown functions
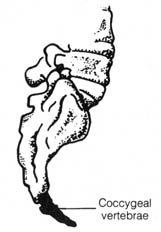 Figure 2.39. Coccygeal vertebrae in humans. Reprinted, with
permission, from Villee, C. A. Biology. 7th ed. © 1977 W. B.
Saunders Co.
Figure 2.39. Coccygeal vertebrae in humans. Reprinted, with
permission, from Villee, C. A. Biology. 7th ed. © 1977 W. B.
Saunders Co.
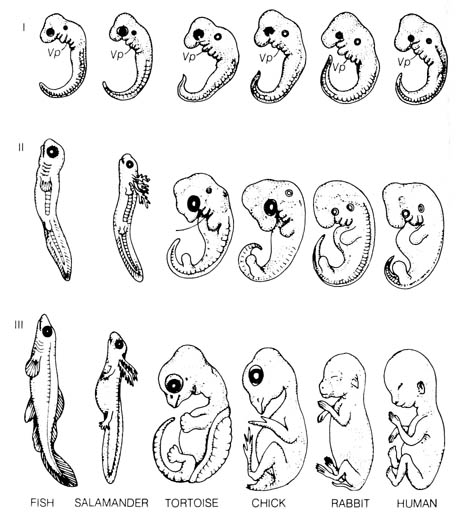
Figure 2.40. A comparison of vertebrate embryos at three
stages of development. Vp (Visceral pouch). From Romanes, G. G.
Darwin
after Darwin. Open Court Publishing Co.; 1910. Recent evidence uncovered
that there were errors in the original drawings of early embryos made by
the German evolutionist Ernst Haeckel (1834-1919). Haeckel faked them in
support of Darwinian evolution by exaggerating their similarities and minimizing
their differences.(16) [142]
in the higher forms of life, evolutionists are obliged to provide
evidence that will link these structures to the exact counterpart
that was
functional in the presumed ancestors. At the same time
they must establish the functionless
status of the structures at
present. The challenge as yet has not been well heeded, and the correlations
often cited are tentative. Another interpretation of vestigial organs is
that the basic homology in structure and the varying function of these
organs indicates they were [143] constructed (created) on the same fundamental
plan for the different habitats where the organisms adapted.
2.5.3 Comparative Embryological Development. Modern embryology
can be traced to the first systematic presentation of the characteristic
development of animal embryos by Karl Ernst von Baer (1792-1876), who was
a critic of the theory of evolution. His classical Von Baer's law (9)
can be summarized as follows: (1) general characters appear in development
before special characters appear; (2) the less general and then the specialized
characters appear from the general characters; (3) as an animal develops,
its appearance becomes progressively different from the form of other animals;
(4) young or embryonic stages of an animal are very much alike, but the
adult animals are different (Figure 2.40).
Muller (10) and Haeckel (11) incorporated
von Baer's observation into what is known as the biogenetic law. The
essence of this law is the assumption that ontogeny (the embryological
development of an individual) recapitulates phylogeny (the evolutionary
ancestry of the individual). In other words the embryo undergoes morphological
changes that resemble the adult forms of its evolutionary ancestors. Muller
cited as evidence of this thesis observations on the development of crustaceans
(10).
The larval forms of several stages of crustaceans closely resemble the
adults in a sequence from primitive to advanced. The larvae were found
to pass through these stages at successive molts, with larvae of primitive
crustaceans stopping early in the series and advanced forms going through
most of the stages.
This interpretation of the biogenetic law is not borne out, however,
by subsequent observations. Most of the developmental stages in the embryo
in higher organisms do not resemble the adult forms of their presumed
evolutionary ancestors. Many of the presumed stages in the evolutionary
lineages are missing in ontogeny. In fact, some of the presumed evolutionary
sequence was observed to be reversed in ontogeny. For example, teeth were
supposed to be developed from modified scales and were probably needed
for biting before the tongue evolved. However, in mammalian embryos the
tongue develops before the teeth (12).
Extensive studies in echinoderm embryology also suggested that the differences
in the larval stages actually have adaptive values, for they contribute
directly to the various distinctive features of the adult forms (13).
In other words, the developmental stages resemble their adult stages more
than the adult stages resemble their presumed ancestors (14).
Therefore, the embryological development of each organism has more to do
with the preparation for its own adult life than with the recapitulation
of its phylogeny. The visceral pouches (pharyngeal clefts) of the embryos
[144] of reptiles, birds, and mammals closely resemble those of the fishes
(Stage I, Figure 2.40) but they actually have no relationship with the
gill slits of the adult fish that developed from these embryonic structures.
In half of the vertebrates, none of the pouches ever bear gills. In the
other half, gills develop only from some of the posterior pouches. No true
internal gills are present in any vertebrate at the embryonic stage in
which the pharyngeal clefts are seen (15).
In light of these difficulties, the biogenetic law has been reinterpreted
to state that embryonic stages resemble embryonic stages of the
ancestral forms and only incidentally do they resemble adult forms. This
has reduced the law to barely stating the obvious since all vertebrate
embryos are known to undergo essentially similar processes of earlier development
that lead to the morphological similarities in Stage I (Figure 2.40). This
pattern of development must be important to the subsequent adult life before
differentiation of the tissues can occur. Consequently, theories of design
or evolution are equally applicable to this evidence.



References 2.5
1. Romer, A. S. Vertebrate paleontology.
Chicago: Univ. Chicago Press; 1966: 79.
2. Sussdorf, D. H.; Draper, L. R.
J.
Infect. Dis. 99:129; 1956.
3. Archer, O. K.; Sutherland, D. R.; Good,
R.A.
Nature. 200:337; 1963.
4. Wurtman, R. J.; Axelrod, J. A.
Sc.
Am. 213 (July):54; 1965.
5. Gaston, S.; Menaker, M.
Science.
160 (June 7):1125; 1968.
6. Thompson, W. R.
Introduction to the
origin of species by C. Darwin. London: J. M. Dent and Sons; 1956:
XIV.
7. Kent, G. C.
Comparative anatomy of
the vertebrate. 3rd ed. St. Louis: Mosby; 1973: 281.
8. Barren, J. T.
Textbook of Immunology.
2nd ed. St. Louis: Mosby; 1974: 65.
9. Von Baer, K. E.
Uber Entwicklungsgeschichte
der Tiere, Beobachtung und Reflexion. Konigsberg; 1828.
10. Muller, F.
Fur Darwin. Leipzig;
1864.
11. Haeckel, E.
Naturliche Schopfungsgeschichte
Berlin; 1868.
12. DeBeer, G.
Embryos and ancestors.
3rd ed. London: Oxford Univ. Press; 1958: 7.
13. Foil, H. B.
Biograph. Rev. 23:81;
1948.
14. Olson, E. C.
The evolution of life.
London: Weidenfeld & Nicholson, 1965; reviewed by DeBeer, G.
Nature.
206 (April 24):331; 1965.
15. Ballard, W. W.
Comparative anatomy
and embryology. New York: Ronald; 1964: 75.
16. Wells, J. Icons of Evolution. Washington DC:
Regnery; 2000, Chap. 5, p. 81-109.













